Lewis Dot Structures: Sigma & Pi Bonds definitions Flashcards
 Back
BackLewis Dot Structures: Sigma & Pi Bonds definitions
1/11
Terms in this set (11)
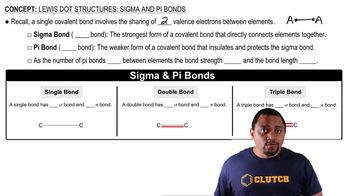
- Covalent bondA chemical bond involving the sharing of two valence electrons between atoms.
- Valence electronsElectrons in the outermost shell of an atom that are involved in forming bonds.
- Sigma bondThe strongest type of covalent bond that directly connects two atoms.
- Pi bondA weaker covalent bond that reinforces and protects a sigma bond.
- Single bondA bond consisting of one sigma bond and no pi bonds.
- Double bondA bond consisting of one sigma bond and one pi bond.
- Triple bondA bond consisting of one sigma bond and two pi bonds.
- Bond strengthThe measure of how strong a bond is, increasing with more pi bonds.
- Bond lengthThe distance between two bonded atoms, decreasing with more pi bonds.
- Molecular structureThe arrangement of atoms within a molecule, influenced by bond types.
- Bond insulationThe protective effect of pi bonds on a sigma bond.