Lewis Dot Structures: Neutral Compounds definitions Flashcards
 Back
BackLewis Dot Structures: Neutral Compounds definitions
1/15
Terms in this set (15)
- Lewis Dot StructureA diagram showing the bonding between atoms and the lone pairs of electrons in a molecule.
- Valence ElectronsElectrons in the outermost shell of an atom, determining its ability to bond with other atoms.
- ElectronegativityA measure of an atom's ability to attract and hold onto electrons in a chemical bond.
- Octet RuleA principle stating that atoms tend to bond in a way that gives them eight electrons in their valence shell.
- Formal ChargeThe charge assigned to an atom in a molecule, assuming equal sharing of electrons in bonds.
- Single BondA chemical bond where one pair of electrons is shared between two atoms.
- Double BondA chemical bond where two pairs of electrons are shared between two atoms.
- Triple BondA chemical bond where three pairs of electrons are shared between two atoms.
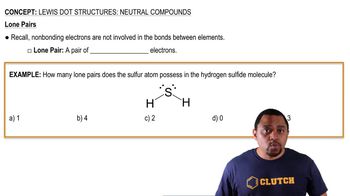
- Lone PairA pair of valence electrons not shared with another atom and not involved in bonding.
- Central AtomThe atom in a molecule that is bonded to multiple other atoms, often the least electronegative.
- Noble GasElements in group 18 of the periodic table, known for having full valence electron shells.
- HalogensElements in group 17 of the periodic table, typically forming single bonds in molecules.
- Nonbonding ElectronsElectrons in an atom's valence shell that are not involved in forming bonds.
- StabilityThe tendency of a molecule to maintain its structure and resist changes in its electron configuration.
- HeliumA noble gas with a full valence shell of two electrons, serving as a model for hydrogen's electron configuration.