Law of Conservation of Mass definitions Flashcards
 Back
BackLaw of Conservation of Mass definitions
1/11
Terms in this set (11)
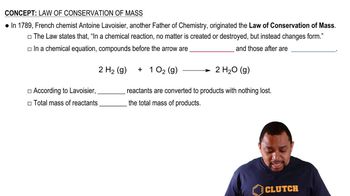
- Antoine LavoisierA French chemist known as the father of modern chemistry who established the law of conservation of mass.
- Conservation of MassA principle stating that mass is neither created nor destroyed in a chemical reaction.
- Chemical ReactionA process where reactants are transformed into products without any loss of mass.
- ReactantsSubstances present before a chemical reaction that are transformed into products.
- ProductsSubstances formed as a result of a chemical reaction from reactants.
- StoichiometryThe calculation of reactants and products in chemical reactions based on the conservation of mass.
- Solution ChemistryA branch of chemistry focusing on the properties and behavior of solutions, guided by mass conservation.
- Balanced Chemical EquationAn equation representing a chemical reaction with equal mass on both sides, reflecting mass conservation.
- H2OThe chemical formula for water, a product formed from hydrogen and oxygen in a reaction.
- H2The chemical formula for hydrogen, a reactant in the formation of water.
- O2The chemical formula for oxygen, a reactant in the formation of water.