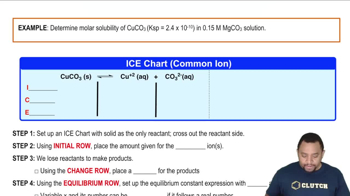
Ksp: Common Ion Effect definitions Flashcards
 Back
BackKsp: Common Ion Effect definitions
1/11
Terms in this set (11)
- Common Ion EffectReduces solubility of a solid in a solution containing ions similar to those in the solid.
- Le Chatelier's PrincipleStates that a system in equilibrium will adjust to minimize the impact of changes.
- Solubility Product ConstantRepresents the maximum amount of an ionic solid that can dissolve in a solution.
- EquilibriumA state where the forward and reverse reactions occur at the same rate.
- Barium SulfideAn ionic compound that dissociates into barium and sulfide ions in solution.
- Barium IonA positively charged ion resulting from the dissociation of barium compounds.
- Sulfide IonA negatively charged ion resulting from the dissociation of sulfide compounds.
- Hydroxide IonA negatively charged ion that decreases the solubility of bases in solution.
- Hydronium IonA positively charged ion that decreases the solubility of acids in solution.
- Pure WaterA solvent with no dissolved ions, allowing maximum solubility of ionic solids.
- Reverse ReactionThe process where products convert back into reactants in a chemical equilibrium.