Introduction to Quantum Mechanics definitions Flashcards
 Back
BackIntroduction to Quantum Mechanics definitions
1/12
Terms in this set (12)
- Quantum MechanicsA theoretical framework describing electron behavior at the atomic scale.
- Quantum NumbersValues specifying energy levels and positions of electrons in an atom.
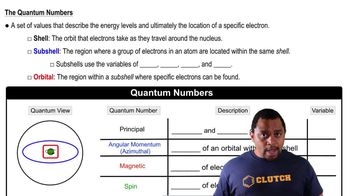
- Principal Quantum NumberIndicates the energy and size of an atomic shell, denoted by n.
- Angular Momentum Quantum NumberDefines the shape of an orbital within a subshell, denoted by l.
- Magnetic Quantum NumberProvides the orientation of an orbital, denoted by m sub l.
- Spin Quantum NumberDetermines the direction of an electron's spin, denoted by m sub s.
- ShellThe orbit that electrons take around the nucleus of an atom.
- SubshellRegions within a shell where electrons are located, labeled s, p, d, f.
- OrbitalRegion within a subshell where specific electrons can be found.
- Electron SpinThe direction of an electron's rotation, either clockwise or counterclockwise.
- Schrodinger's Wave EquationA complex mathematical equation used to describe electron behavior.
- Azimuthal Quantum NumberAnother term for angular momentum quantum number, defining orbital shape.