Intro to Addition Reactions definitions Flashcards
 Back
BackIntro to Addition Reactions definitions
1/12
Terms in this set (12)
- AlkeneA hydrocarbon with at least one carbon-carbon double bond, undergoing addition reactions.
- AlkyneA hydrocarbon with at least one carbon-carbon triple bond, capable of addition reactions.
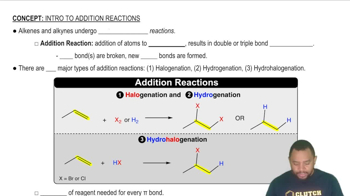
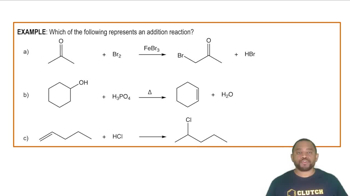
- Addition ReactionA process where atoms are added to pi bonds, breaking double or triple bonds.
- Pi BondA type of covalent bond formed by the sideways overlap of p orbitals, present in double and triple bonds.
- Sigma BondA covalent bond formed by the head-on overlap of atomic orbitals, present in all single bonds.
- HalogenationAn addition reaction where halogens are added to pi bonds, forming dihalides.
- HydrogenationAn addition reaction where hydrogen atoms are added to pi bonds, converting alkenes to alkanes.
- HydrohalogenationAn addition reaction where a hydrogen and a halogen are added to an alkene, forming alkyl halides.
- DihalideA compound formed by the addition of two halogen atoms to a molecule.
- Alkyl HalideA compound formed by the addition of a hydrogen and a halogen to an alkene.
- ReagentA substance or compound added to a system to cause a chemical reaction.
- MoleA unit of measurement for amount of substance, used to quantify reagents in reactions.