Intensive vs. Extensive Properties definitions Flashcards
 Back
BackIntensive vs. Extensive Properties definitions
1/13
Terms in this set (13)
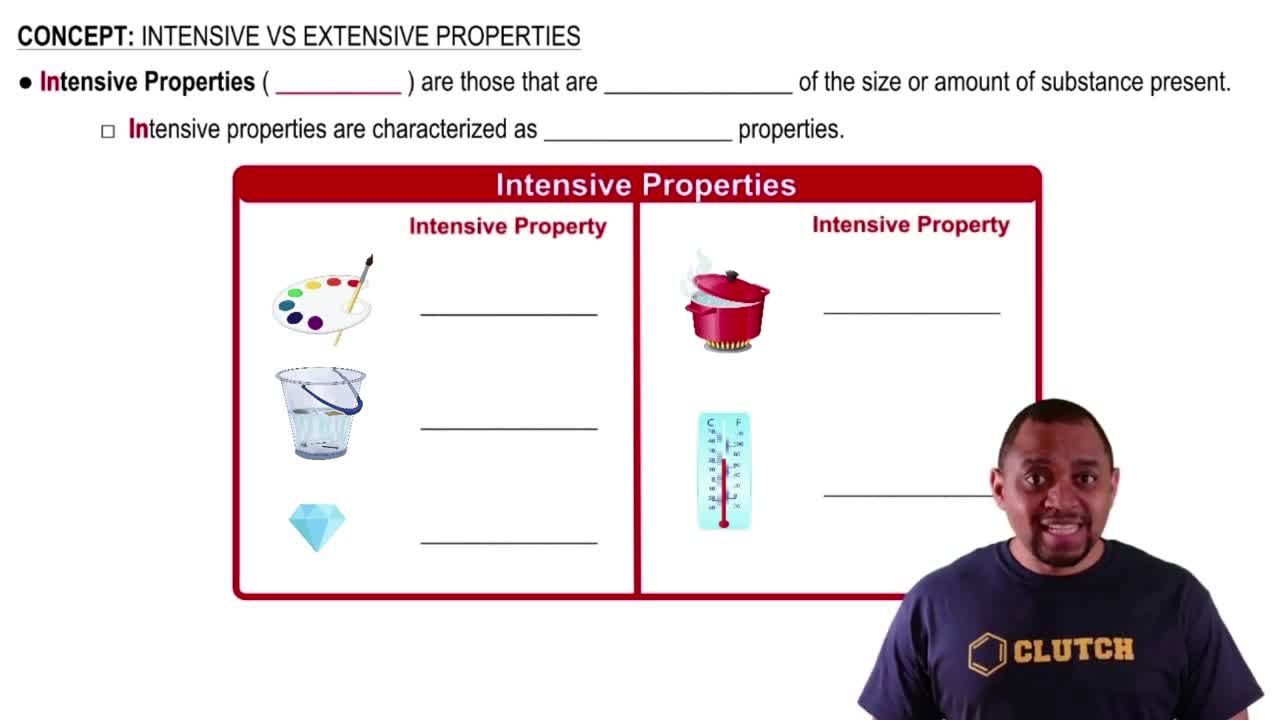
- Intensive propertiesPhysical characteristics of a substance that do not change with the amount present, such as color and density.
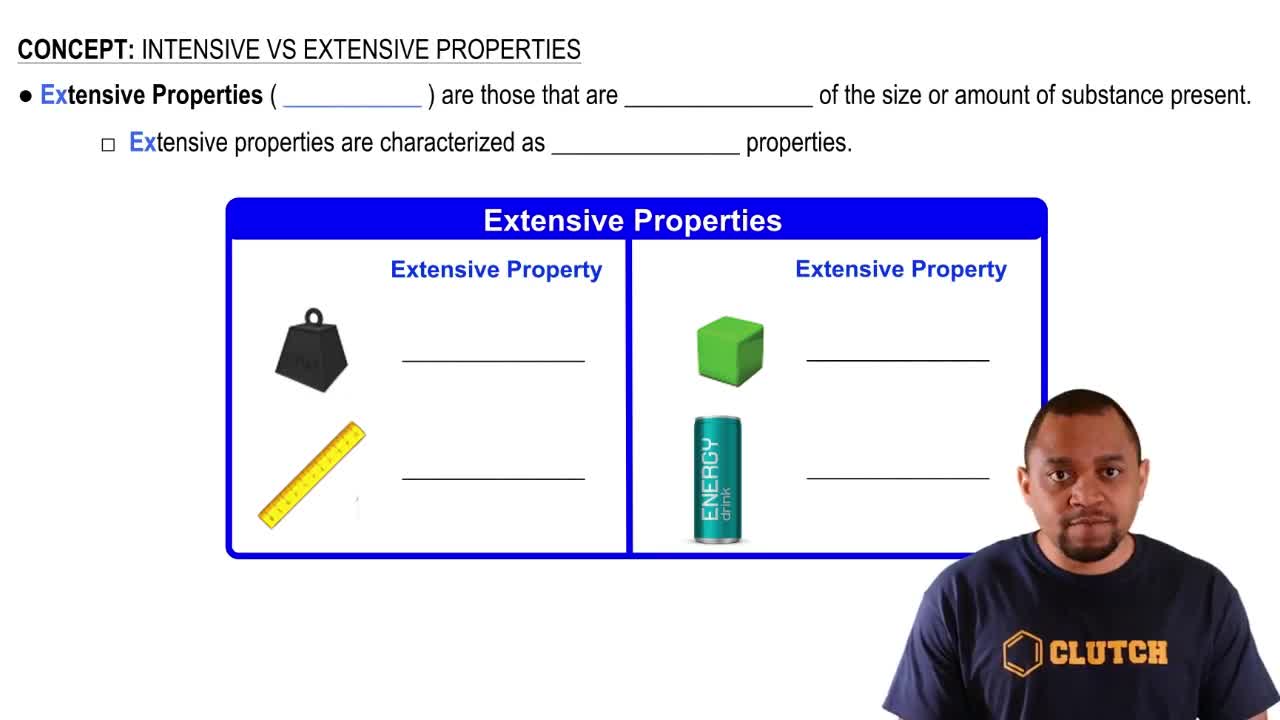
- Extensive propertiesPhysical characteristics that vary with the amount of substance, like mass and volume.
- DensityA measure of mass per unit volume, remaining constant regardless of the amount of substance.
- Boiling pointThe temperature at which a liquid turns to vapor, unaffected by the quantity of the liquid.
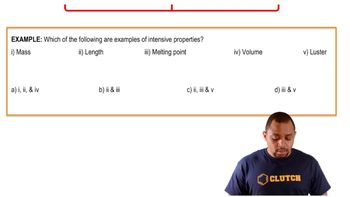
- Melting pointThe temperature at which a solid becomes a liquid, independent of the amount of the substance.
- Freezing pointThe temperature at which a liquid becomes a solid, not influenced by the volume of the liquid.
- TemperatureA measure of thermal energy that remains constant for a substance regardless of its quantity.
- MassThe amount of matter in an object, which changes with the quantity of the substance.
- VolumeThe amount of space occupied by a substance, varying with the amount present.
- LengthA measure of distance, which changes based on the amount of material.
- EnergyThe capacity to do work, dependent on the amount of substance, including forms like thermal and nuclear.
- ColorA visual attribute of a substance that remains unchanged regardless of the amount present.
- HardnessA measure of a material's resistance to deformation, constant regardless of the quantity.