Instantaneous Rate definitions Flashcards
 Back
BackInstantaneous Rate definitions
1/10
Terms in this set (10)
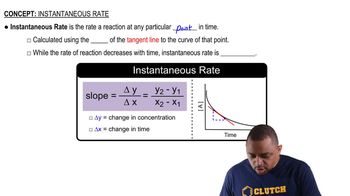
- Instantaneous RateThe rate of a reaction at a specific moment, determined by the slope of the tangent line on a concentration vs. time graph.
- Tangent LineA straight line that touches a curve at a single point, used to determine the instantaneous rate of a reaction.
- SlopeA measure of the steepness of a line, calculated as the change in y over the change in x, used to find the instantaneous rate.
- ConcentrationThe amount of a substance in a given volume, which decreases as a reaction progresses, affecting the reaction rate.
- Reaction KineticsThe study of the rates of chemical reactions and the factors affecting them, including instantaneous rate.
- Rate LawsMathematical expressions that describe the relationship between the rate of a reaction and the concentration of reactants.
- Reaction MechanismsThe step-by-step sequence of elementary reactions by which overall chemical change occurs, influenced by instantaneous rate.
- Graphical RepresentationA visual depiction of data, such as a concentration vs. time graph, used to analyze reaction rates.
- Change in ConcentrationThe difference in the amount of reactant or product over time, used to calculate the slope for instantaneous rate.
- Change in TimeThe interval over which a reaction is observed, used in calculating the slope for determining instantaneous rate.