Hydrohalogenation Reactions definitions Flashcards
 Back
BackHydrohalogenation Reactions definitions
1/15
Terms in this set (15)
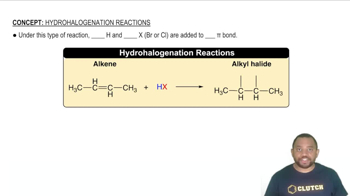
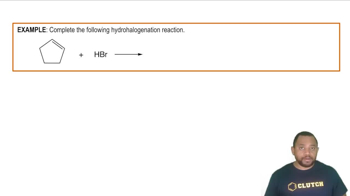
- HydrohalogenationA reaction where hydrogen and a halogen are added across a pi bond of an alkene or alkyne.
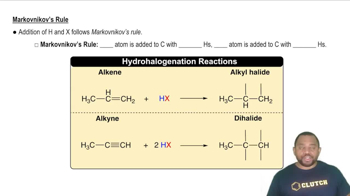
- AlkeneA hydrocarbon containing a carbon-carbon double bond.
- AlkyneA hydrocarbon containing a carbon-carbon triple bond.
- Alkyl HalideA compound formed when a halogen is added to an alkane, alkene, or alkyne.
- Markovnikov's RuleA rule stating that hydrogen adds to the carbon with more hydrogens in unsymmetrical alkenes or alkynes.
- Pi BondA type of covalent bond formed by the sideways overlap of atomic orbitals.
- Symmetrical MoleculeA molecule where substituents are evenly distributed, allowing equal addition of atoms.
- DihalideA compound with two halogen atoms added to the same carbon.
- HydrogenThe lightest and most abundant element, often added in hydrohalogenation reactions.
- HalogenA group 17 element, such as bromine or chlorine, used in hydrohalogenation.
- BromineA halogen element commonly used in hydrohalogenation reactions.
- ChlorineA halogen element often used in hydrohalogenation reactions.
- Double BondA chemical bond where two pairs of electrons are shared between atoms.
- Triple BondA chemical bond where three pairs of electrons are shared between atoms.
- MoleA unit of measurement for amount of substance, used to quantify reactants in reactions.