Hybridization definitions Flashcards
 Back
BackHybridization definitions
1/14
Terms in this set (14)
- HybridizationMixing of atomic orbitals to form new hybrid orbitals for bond formation and stability.
- Atomic OrbitalsRegions in an atom where electrons are likely to be found, such as s and p orbitals.
- Hybrid OrbitalsOrbitals formed from the combination of atomic orbitals, resembling a p orbital with unequal lobes.
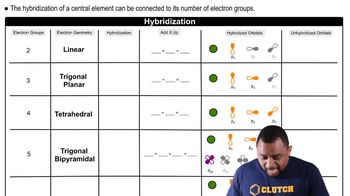
- Electron GroupsRegions of electron density around a central atom, influencing molecular shape and hybridization.
- Electron GeometryThe spatial arrangement of electron groups around a central atom, determining molecular shape.
- Linear GeometryMolecular shape with two electron groups, associated with sp hybridization.
- Trigonal PlanarMolecular shape with three electron groups, associated with sp2 hybridization.
- TetrahedralMolecular shape with four electron groups, associated with sp3 hybridization.
- Trigonal BipyramidalMolecular shape with five electron groups, associated with sp3d hybridization.
- OctahedralMolecular shape with six electron groups, associated with sp3d2 hybridization.
- Unhybridized OrbitalsAtomic orbitals that remain unchanged during hybridization, such as remaining p or d orbitals.
- s OrbitalSpherical atomic orbital involved in hybridization, contributing to hybrid orbitals.
- p OrbitalsDumbbell-shaped atomic orbitals, three of which can participate in hybridization.
- d OrbitalsComplex-shaped atomic orbitals, five in total, some of which can be hybridized.