Hess's Law definitions Flashcards
 Back
BackHess's Law definitions
1/15
Terms in this set (15)
- Hess's LawA principle stating that the total enthalpy change of a reaction is the same, regardless of the pathway taken.
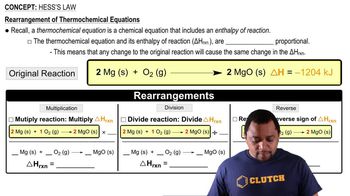
- Thermochemical EquationA chemical equation that includes the enthalpy change of the reaction.
- Enthalpy ChangeThe heat change associated with a chemical reaction, denoted as ΔH.
- Reaction PathwayThe series of steps or reactions that lead to the final product in a chemical process.
- CoefficientsNumbers placed before reactants and products in a chemical equation to balance it.
- Reaction IntermediatesSpecies that appear in the steps of a reaction mechanism but not in the overall balanced equation.
- Standard EnthalpyThe enthalpy change when all reactants and products are in their standard states.
- Reversing ReactionChanging the direction of a chemical reaction, which reverses the sign of ΔH.
- Partial ReactionAn individual step in a multi-step reaction process.
- Overall ReactionThe net result of combining all steps in a reaction pathway.
- KilojoulesA unit of energy used to express enthalpy changes in reactions.
- Magnesium OxideA compound formed from the reaction of magnesium with oxygen.
- Xenon DifluorideA chemical compound used as a reactant in certain thermochemical equations.
- Xenon TrifluorideA chemical compound that can be a product in thermochemical reactions.
- FluorineA diatomic molecule that can act as a reactant or product in chemical reactions.