Intro to Henry's Law definitions Flashcards
 Back
BackIntro to Henry's Law definitions
1/11
Terms in this set (11)
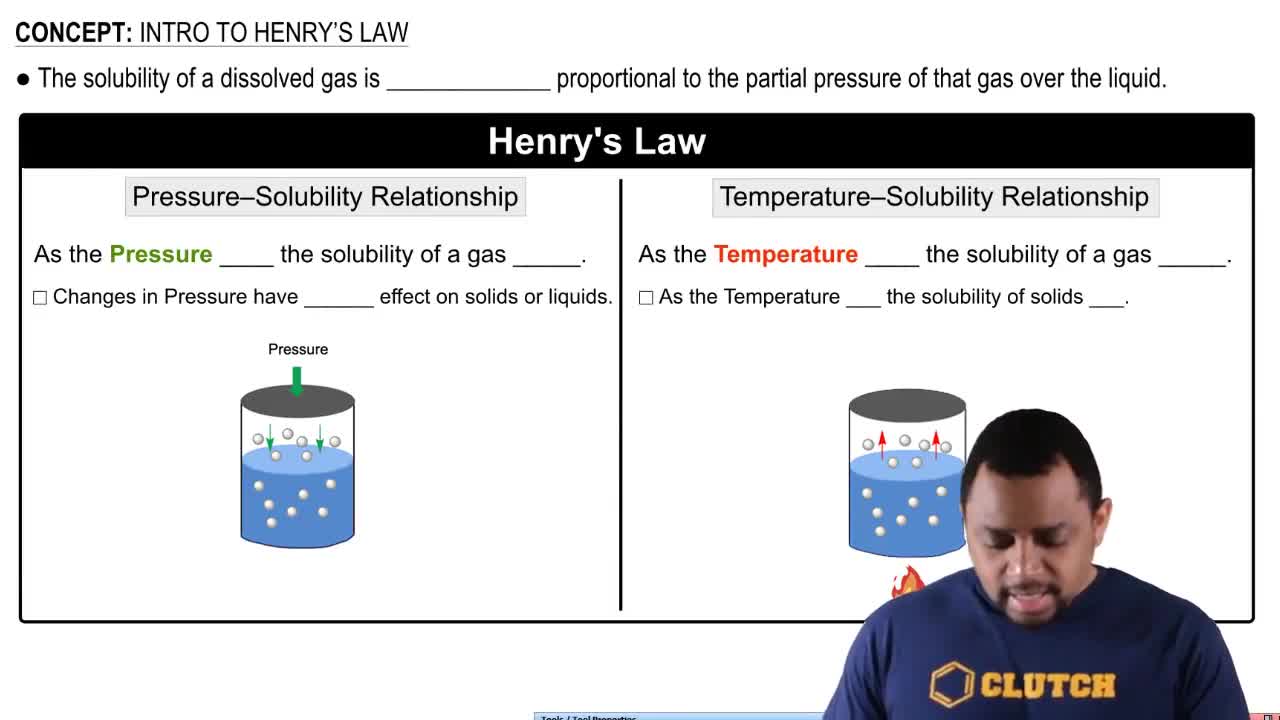
- Henry's LawDescribes the proportional relationship between gas solubility in a liquid and the gas's partial pressure above the liquid.
- SolubilityThe ability of a solute to dissolve in a solvent, influenced by pressure and temperature.
- Partial PressureThe pressure exerted by a single type of gas in a mixture of gases.
- Pressure-Solubility RelationshipAs pressure increases, the solubility of a gas in a liquid increases.
- Temperature-Solubility RelationshipAs temperature increases, gas solubility decreases, while solid solubility increases.
- Gas SolubilityThe extent to which a gas can dissolve in a liquid, affected by pressure and temperature.
- Solid SolubilityThe extent to which a solid can dissolve in a liquid, typically increasing with temperature.
- Closed ContainerA sealed environment where pressure can be applied to affect gas solubility.
- SteamWater vapor that forms when water is heated, indicating decreased gas solubility.
- SolventThe liquid in which a solute is dissolved to form a solution.
- SoluteThe substance that is dissolved in a solvent to form a solution.