Henry's Law Calculations definitions Flashcards
 Back
BackHenry's Law Calculations definitions
1/11
Terms in this set (11)
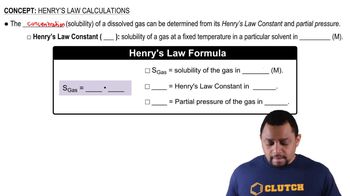
- Henry's LawDescribes the relationship between gas solubility and its partial pressure.
- SolubilityThe concentration of a dissolved gas in a solvent, often measured in molarity.
- Partial PressureThe pressure exerted by a single gas in a mixture of gases.
- MolarityA measure of concentration, representing moles of solute per liter of solution.
- Henry's Law ConstantA proportionality constant in Henry's Law, indicating solubility at a fixed temperature.
- AtmospheresA unit of pressure commonly used in expressing gas pressures.
- TorrA unit of pressure equivalent to 1/760 of an atmosphere.
- Millimeters of MercuryA unit of pressure also known as mmHg, used in measuring gas pressures.
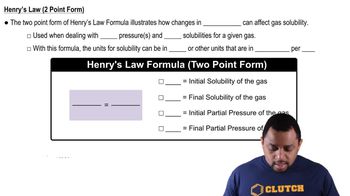
- S1P1=S2P2A formula used to compare solubility and pressure under two different conditions.
- TemperatureA factor affecting the value of Henry's Law constant.
- SolventThe liquid in which a gas is dissolved to form a solution.