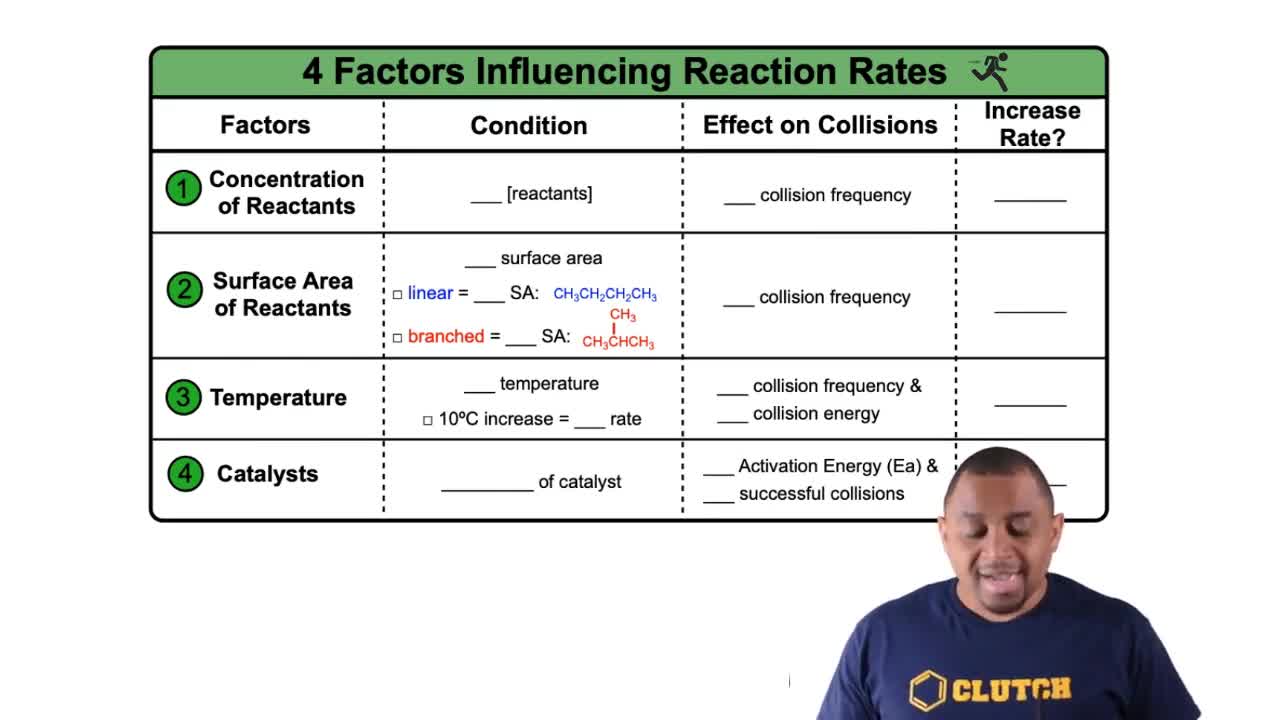
Factors Influencing Rates definitions Flashcards
 Back
BackFactors Influencing Rates definitions
1/12
Terms in this set (12)
- Collision FrequencyThe number of molecular collisions occurring per unit of time, influencing reaction rates.
- Successful CollisionsEnergetic molecular collisions that result in product formation during a reaction.
- Reactant ConcentrationThe amount of reactants in a mixture, affecting the likelihood of molecular collisions.
- Surface AreaThe extent of a reactant's exposed area, impacting collision frequency and reaction rate.
- Linear CompoundsMolecules with a straight chain structure, offering higher surface area for reactions.
- Branched CompoundsMolecules with side chains, reducing surface area compared to linear structures.
- TemperatureA measure of thermal energy that affects molecular speed and collision energy.
- Activation EnergyThe minimum energy required for reactants to transform into products in a reaction.
- CatalystA substance that lowers activation energy, increasing the rate of a chemical reaction.
- Reaction RateThe speed at which reactants are converted into products in a chemical reaction.
- Collision EnergyThe energy possessed by colliding molecules, crucial for successful reactions.
- Thermal EnergyEnergy from heat that increases molecular motion and collision frequency.