Ester Reactions: Saponification definitions Flashcards
 Back
BackEster Reactions: Saponification definitions
1/10
Terms in this set (10)
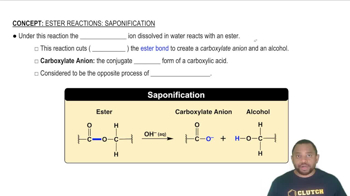
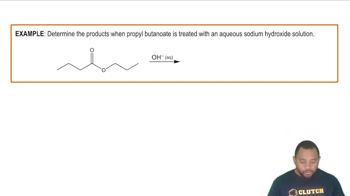
- SaponificationA reaction where hydroxide ions cleave an ester bond, forming a carboxylate anion and an alcohol.
- Hydroxide ionAn ion with a negative charge, OH-, that reacts with esters in saponification.
- EsterA compound that reacts with hydroxide ions in saponification to form a carboxylate anion and alcohol.
- Carboxylate anionThe negatively charged conjugate base of a carboxylic acid formed in saponification.
- AlcoholA product of saponification formed when an ester bond is cleaved by hydroxide ions.
- Conjugate baseA species formed by the loss of a proton (H+) from a carboxylic acid, resulting in a negative charge.
- EsterificationThe process opposite to saponification, where an ester is formed from an alcohol and a carboxylic acid.
- Carboxylic acidAn acid that loses an H+ to form a carboxylate anion during saponification.
- AqueousA term describing a solution where water is the solvent, as in aqueous OH- used in saponification.
- CarbonylA functional group consisting of a carbon atom double-bonded to an oxygen atom, involved in ester bonds.