Entropy definitions Flashcards
 Back
BackEntropy definitions
1/15
Terms in this set (15)
- EntropyA measure of disorder or randomness in a system, increasing with molecular complexity, temperature, and number of moles.
- ThermodynamicsThe study of the relationship between heat, energy, and reaction favorability.
- Second Law of ThermodynamicsStates that the entropy of the universe always increases in spontaneous processes.
- Standard Molar EntropyThe entropy possessed by 1 mole of a substance at standard conditions (25°C and 1 atm).
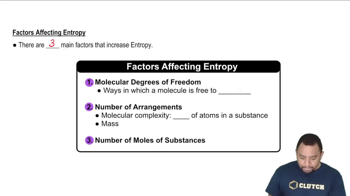
- Molecular Degrees of FreedomThe ways in which a molecule can move, affecting its entropy.
- Molecular ComplexityThe number of atoms in a substance, influencing its entropy.
- Phase TransitionThe change of a substance from one state of matter to another, affecting its entropy.
- ChaosA synonym for disorder or randomness, often used to describe entropy.
- Spontaneous ReactionA reaction that occurs naturally, increasing the entropy of the universe.
- MolesA measure of substance amount, where more moles indicate higher entropy.
- TemperatureA factor that affects molecular motion and entropy, with higher temperatures increasing entropy.
- PressureA factor that affects molecular spacing and entropy, with lower pressures increasing entropy.
- Energy TransformationThe conversion of energy from one form to another, often resulting in some energy lost as entropy.
- DisorderThe lack of order or predictability, synonymous with entropy.
- Chemical ReactionA process that involves rearrangement of molecular or ionic structure, affecting entropy.