Empirical Formula definitions Flashcards
 Back
BackEmpirical Formula definitions
1/12
Terms in this set (12)
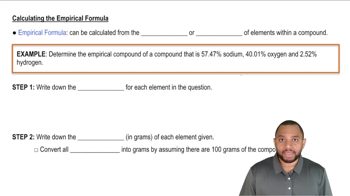
- Empirical FormulaRepresents the simplest whole number ratio of elements in a compound, derived from mass percentages.
- Molecular FormulaIndicates the exact number of atoms of each element within a compound.
- Mole ConceptA method to convert between grams, moles, and units like molecules, ions, or atoms.
- Mass PercentageThe percentage by mass of each element in a compound.
- Whole Number RatioA ratio where the numbers are whole numbers, used in empirical formulas.
- Constituent ElementsThe individual elements that make up a compound.
- Simplified FormA reduced version of a formula showing the smallest whole number ratio.
- C6H12O6An example of a molecular formula that can be reduced to an empirical formula.
- CH2OThe empirical formula derived from C6H12O6 by dividing by a common factor.
- C3H6O3A molecular formula that can be reduced to CH2O as its empirical formula.
- C10H14N2A molecular formula that reduces to C5H7N as its empirical formula.
- C12H22O11A molecular formula that cannot be reduced, so it is also its empirical formula.