Electron Capture & Positron Emission definitions Flashcards
 Back
BackElectron Capture & Positron Emission definitions
1/12
Terms in this set (12)
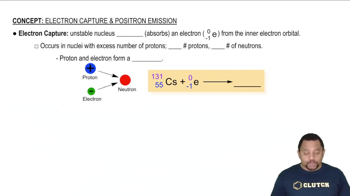
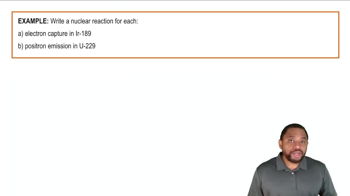
- Electron CaptureA process where an unstable nucleus absorbs an electron, converting a proton into a neutron, reducing proton count.
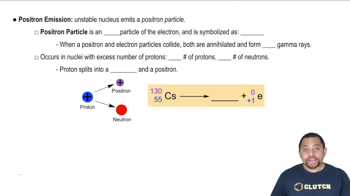
- Positron EmissionA process where a proton transforms into a neutron, emitting a positron, reducing the proton count.
- ProtonA subatomic particle found in the nucleus with a positive charge, its number defines the element.
- NeutronA subatomic particle in the nucleus with no charge, contributing to the atomic mass.
- PositronAn antiparticle of the electron with a positive charge and no mass number, emitted during positron emission.
- AntiparticleA particle with the same mass as another particle but opposite charge, such as a positron to an electron.
- Gamma RaysHigh-energy electromagnetic radiation emitted during particle annihilation, such as electron-positron collisions.
- Mass NumberThe total number of protons and neutrons in an atomic nucleus, remains constant in nuclear reactions.
- Atomic NumberThe number of protons in an atom's nucleus, determining the element's identity.
- XenonA noble gas element formed from cesium through electron capture or positron emission.
- CesiumA chemical element that undergoes electron capture or positron emission to form xenon.
- NucleusThe central part of an atom containing protons and neutrons, where nuclear reactions occur.