Electrolytic Cell definitions Flashcards
 Back
BackElectrolytic Cell definitions
1/15
Terms in this set (15)
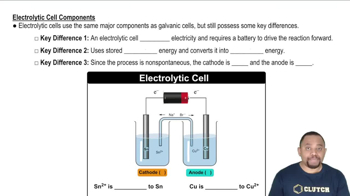
- Electrolytic CellA type of electrochemical cell that uses external electrical energy to drive non-spontaneous reactions.
- ElectrolysisA process where chemical reactions consume external electrical energy to occur.
- CathodeThe electrode where reduction occurs, negatively charged in an electrolytic cell.
- AnodeThe electrode where oxidation occurs, positively charged in an electrolytic cell.
- Redox ReactionA chemical reaction involving the transfer of electrons between two species.
- BatteryA device that provides the external electrical energy needed for electrolysis in an electrolytic cell.
- Standard Cell PotentialA measure of the voltage difference between two half-cells in an electrochemical cell.
- Gibbs Free EnergyA thermodynamic quantity indicating the amount of energy available to do work.
- EntropyA measure of the disorder or randomness in a system.
- Equilibrium Constant (K)A ratio of the concentrations of products to reactants at equilibrium.
- Reaction Quotient (Q)A ratio of the concentrations of products to reactants at any point in a reaction.
- Non-spontaneous ReactionA reaction that does not occur naturally and requires external energy to proceed.
- Salt BridgeA device used to maintain electrical neutrality by allowing ions to flow between two half-cells.
- Lithium BatteryA type of rechargeable battery that uses lithium ions to store and release energy.
- Rechargeable BatteryA battery that can be recharged and used multiple times by reversing the chemical reaction.