Diprotic Acids and Bases Calculations definitions Flashcards
 Back
BackDiprotic Acids and Bases Calculations definitions
1/12
Terms in this set (12)
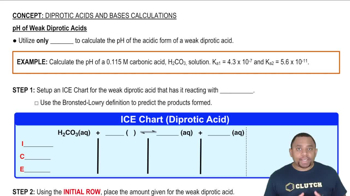
- Diprotic AcidAn acid capable of donating two protons or hydrogen ions per molecule in aqueous solutions.
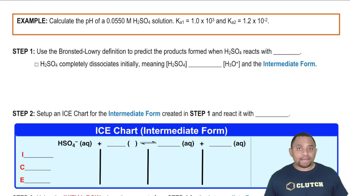
- Sulfuric AcidThe only strong diprotic acid, fully dissociating its first proton in water.
- HSO4-The bisulfate ion formed after sulfuric acid loses its first proton.
- H3O+The hydronium ion formed when an acid donates a proton to water.
- DissociationThe process by which an acid breaks down into ions in a solution.
- K1The dissociation constant for the first proton of a diprotic acid.
- K2The dissociation constant for the second proton of a diprotic acid.
- Reversible ArrowsIndicate a reaction where reactants partially convert to products.
- Sulfate IonThe ion formed after sulfuric acid loses both of its protons.
- pHA measure of the acidity or basicity of an aqueous solution.
- Acidic ProtonA hydrogen ion that an acid can donate in a reaction.
- Aqueous SolutionA solution in which water is the solvent.