Crystal Field Theory: Tetrahedral Complexes definitions Flashcards
 Back
BackCrystal Field Theory: Tetrahedral Complexes definitions
1/15
Terms in this set (15)
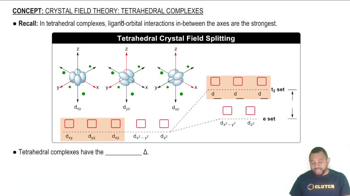
- Tetrahedral ComplexA molecular structure where four ligands are symmetrically arranged around a central atom.
- LigandAn ion or molecule that binds to a central metal atom to form a coordination complex.
- d OrbitalA type of atomic orbital with specific shapes and orientations, crucial in transition metal chemistry.
- dxy OrbitalA d orbital oriented between the x and y axes, experiencing strong ligand interactions.
- dyz OrbitalA d orbital oriented between the y and z axes, experiencing strong ligand interactions.
- dxz OrbitalA d orbital oriented between the x and z axes, experiencing strong ligand interactions.
- dx2-y2 OrbitalA d orbital oriented along the x and y axes, experiencing weaker ligand interactions.
- dz2 OrbitalA d orbital oriented along the z axis, experiencing weaker ligand interactions.
- Triplet SetA group of three orbitals with the highest energy in a tetrahedral complex.
- Pair SetA group of two orbitals with lower energy in a tetrahedral complex.
- Crystal Field SplittingThe energy difference between sets of d orbitals in a coordination complex.
- DeltaThe symbol representing the energy difference between d orbital sets in a complex.
- Octahedral ComplexA molecular structure where six ligands are symmetrically arranged around a central atom.
- Square Planar ComplexA molecular structure where four ligands are arranged in a square around a central atom.
- Crystal Field DiagramA visual representation of the energy levels of d orbitals in a coordination complex.