Crystal Field Theory Summary definitions Flashcards
 Back
BackCrystal Field Theory Summary definitions
1/15
Terms in this set (15)
- Crystal Field TheoryExplains d-orbital splitting in metal complexes, affecting color and magnetism.
- d-OrbitalsOrbitals involved in bonding and splitting in metal complexes, influencing energy levels.
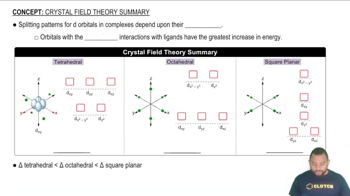
- Tetrahedral ComplexComplex with smallest splitting energy, where dxy, dyz, and dxz orbitals are higher in energy.
- Octahedral ComplexComplex with intermediate splitting, where dx2-y2 and dz2 orbitals are higher in energy.
- Square Planar ComplexComplex with largest splitting energy, where dx2-y2 orbital is at the highest energy.
- Splitting EnergyEnergy difference between higher and lower d-orbital levels in a complex.
- LigandsMolecules or ions that interact with metal orbitals, affecting their energy levels.
- dxy OrbitalOrbital with higher energy in tetrahedral and square planar complexes.
- dyz OrbitalOrbital with higher energy in tetrahedral complexes, lower in square planar.
- dxz OrbitalOrbital with higher energy in tetrahedral complexes, lower in square planar.
- dx2-y2 OrbitalOrbital with highest energy in square planar and octahedral complexes.
- dz2 OrbitalOrbital with intermediate energy in square planar, higher in octahedral.
- AxesReference lines for orbital orientation, affecting interaction with ligands.
- Energy LevelsRelative energies of orbitals in a complex, influenced by ligand interactions.
- InteractionDegree of overlap between orbitals and ligands, affecting energy and splitting.