Crystal Field Theory: Square Planar Complexes definitions Flashcards
 Back
BackCrystal Field Theory: Square Planar Complexes definitions
1/15
Terms in this set (15)
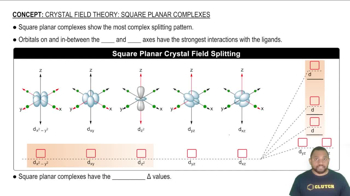
- Square Planar ComplexesCoordination compounds with a unique d-orbital splitting pattern due to ligand interactions on the x and y axes.
- d-OrbitalsAtomic orbitals involved in bonding, exhibiting different energy levels in square planar complexes.
- LigandsMolecules or ions that interact with metal centers, affecting d-orbital energy levels.
- X AxisOne of the axes along which ligands interact strongly with d-orbitals in square planar complexes.
- Y AxisThe other axis along which ligands interact strongly with d-orbitals in square planar complexes.
- d(x2-y2)The highest energy d-orbital in square planar complexes, interacting strongly with x and y axes.
- dxyA d-orbital with significant interaction with both x and y axes, having the second highest energy.
- dz2A d-orbital with moderate interaction with x and y axes, forming a disc shape.
- dyzA d-orbital interacting primarily with the y axis, having lower energy in square planar complexes.
- dxzA d-orbital interacting primarily with the x axis, having lower energy in square planar complexes.
- Crystal Field SplittingThe energy difference between d-orbitals due to ligand interactions in coordination complexes.
- Splitting Energy (Δ)The energy difference between the highest and lowest d-orbitals in a complex.
- Degenerate OrbitalsOrbitals with the same energy level, such as dyz and dxz in square planar complexes.
- Energy GradientThe variation in energy levels of d-orbitals due to differing ligand interactions.
- Coordination GeometryThe spatial arrangement of ligands around a central atom in a complex.