Crystal Field Theory: Octahedral Complexes definitions Flashcards
 Back
BackCrystal Field Theory: Octahedral Complexes definitions
1/15
Terms in this set (15)
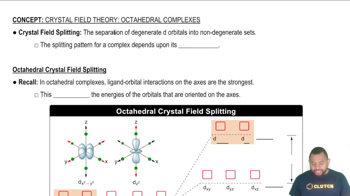
- Crystal Field SplittingThe division of degenerate d orbitals into two energy levels due to ligand interactions.
- Degenerate OrbitalsOrbitals that have the same energy level before splitting occurs.
- Octahedral ComplexA complex ion where ligands are positioned at the corners of an octahedron around a central atom.
- LigandAn ion or molecule that donates a pair of electrons to a central atom in a complex.
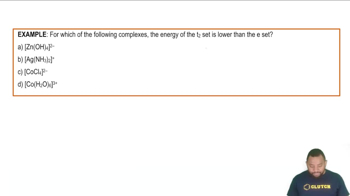
- E SetThe pair of d orbitals (dx²-y² and dz²) with higher energy in an octahedral complex.
- T SetThe triplet of d orbitals with lower energy in an octahedral complex.
- Crystal Field Splitting Energy (Δ)The energy difference between the E set and T set of orbitals in a complex.
- d OrbitalsA set of five orbitals that can be split into different energy levels in a complex.
- GeometryThe spatial arrangement of ligands around the central atom in a complex.
- Energy LevelsThe specific energies that electrons can have in an atom or molecule.
- AxisAn imaginary line that passes through the center of a complex, influencing orbital energy.
- InteractionThe effect of ligands on the energy of d orbitals in a complex.
- DoubletA pair of orbitals in the E set of an octahedral complex.
- TripletA group of three orbitals in the T set of an octahedral complex.
- d x²-y² and d z²The d orbitals oriented along the axes with higher energy in an octahedral complex.