Complex Ions definitions Flashcards
 Back
BackComplex Ions definitions
1/10
Terms in this set (10)
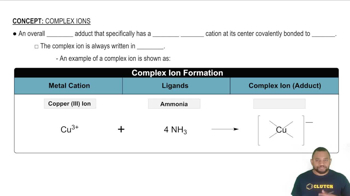
- Complex IonA charged entity with a transition metal cation at its core, covalently bonded to ligands, identifiable by brackets in formulas.
- Transition MetalA metal element that can form cations with an incomplete d electron subshell, often central in complex ions.
- CationA positively charged ion, often a central component in complex ions, formed by losing electrons.
- LigandA molecule or ion that donates a pair of electrons to a metal cation to form a coordinate bond in a complex ion.
- AdductA compound formed from the addition of two or more substances, such as a metal cation and ligands in a complex ion.
- BracketsSymbols used in chemical formulas to enclose complex ions, indicating the group of atoms acting as a single unit.
- ChargeThe electrical property of a complex ion, determined by the sum of the charges of the metal cation and ligands.
- Copper(III) IonA copper ion with a 3+ charge, often forming complex ions with ligands like ammonia.
- AmmoniaA neutral ligand that can bond to metal cations in complex ions, contributing no charge.
- Chemical FormulaA representation of a substance using symbols for its constituent elements, with complex ions in brackets.