Complex Ions: Formation Constant definitions Flashcards
 Back
BackComplex Ions: Formation Constant definitions
1/11
Terms in this set (11)
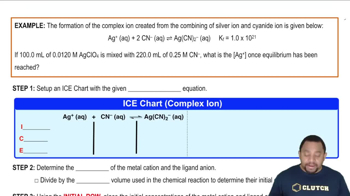
- Complex IonA structure formed when a metal cation bonds with a ligand, acting as a Lewis acid-base pair.
- Metal CationA positively charged metal ion that acts as a Lewis acid by accepting electron pairs.
- Lewis AcidAn electron pair acceptor, often a metal cation in the context of complex ions.
- LigandA molecule or ion that donates a lone pair of electrons, acting as a Lewis base.
- Lewis BaseAn electron pair donor, typically a ligand in complex ion formation.
- AmmoniaA neutral molecule with a lone pair of electrons, often acting as a ligand.
- Cadmium IonA metal cation with a 2+ charge, capable of forming complex ions with ligands.
- Formation ConstantAn equilibrium constant (Kf) expressing the stability of a complex ion in solution.
- Equilibrium ConstantA ratio of product to reactant concentrations, excluding solids and liquids.
- Electron PairA pair of electrons shared or transferred in chemical bonding, crucial in Lewis acid-base reactions.
- Net ChargeThe overall charge of a complex ion, determined by the charges of its components.