Cell Potential: ∆G and K definitions Flashcards
 Back
BackCell Potential: ∆G and K definitions
1/10
Terms in this set (10)
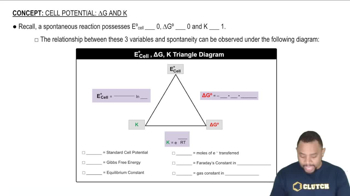
- Spontaneous ReactionA process with a standard cell potential greater than zero and a negative Gibbs free energy change.
- Standard Cell PotentialThe voltage difference between two half-cells in an electrochemical cell under standard conditions.
- Gibbs Free EnergyA thermodynamic quantity representing the amount of energy available to do work.
- Equilibrium ConstantA number that expresses the ratio of products to reactants at equilibrium.
- Nernst EquationAn equation that relates the cell potential to the equilibrium constant and temperature.
- Faraday's ConstantThe charge of one mole of electrons, approximately 96,485 C/mol e-.
- Gas ConstantA physical constant denoted by R, approximately 8.314 J/mol·K.
- TemperatureA measure of the thermal energy within a system, typically in Kelvin for these equations.
- Moles of ElectronsThe quantity of electrons transferred in a redox reaction, denoted by N.
- Triangle DiagramA visual representation connecting standard cell potential, Gibbs free energy, and equilibrium constant.