Cell Potential and Gibbs Free Energy definitions Flashcards
 Back
BackCell Potential and Gibbs Free Energy definitions
1/10
Terms in this set (10)
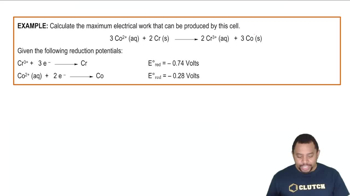
- Gibbs Free EnergyRepresents the maximum electrical work that can be created in an electrochemical cell.
- Standard Cell PotentialThe voltage of an electrochemical cell under standard conditions, measured in volts.
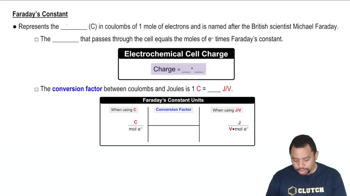
- Faraday's ConstantA constant representing the charge of 1 mole of electrons, approximately 96,485 coulombs.
- Redox ReactionA chemical reaction involving the transfer of electrons from one species to another.
- CoulombA unit of electric charge, where 1 coulomb equals 1 joule per volt.
- VoltThe unit of electric potential, representing the potential difference that drives current.
- KilojouleA unit of energy equal to 1,000 joules, often used to express Gibbs free energy.
- Moles of ElectronsThe quantity of electrons transferred in a redox reaction, denoted by 'n'.
- Michael FaradayA British scientist after whom Faraday's constant is named, known for his work in electromagnetism.
- SpontaneityThe tendency of a process to occur without external influence, related to Gibbs free energy.