Carboxylic Acid Reactions definitions Flashcards
 Back
BackCarboxylic Acid Reactions definitions
1/10
Terms in this set (10)
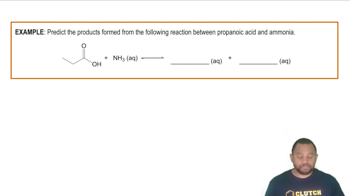
- Carboxylic AcidAn organic compound containing a carboxyl group, known for its weak acidic properties.
- Acid-Base ReactionA chemical process where an acid donates a proton to a base, forming a conjugate base and a conjugate acid.
- BaseA substance that can accept protons from acids, often resulting in the formation of a conjugate base.
- ProtonA positively charged subatomic particle, often represented as H+ in chemical reactions.
- Conjugate BaseThe species formed when an acid donates a proton during an acid-base reaction.
- Carboxylate AnionThe negatively charged ion formed when a carboxylic acid loses a proton.
- Ethanoic AcidA common carboxylic acid, also known as acetic acid, with the formula CH3COOH.
- EthanoateThe conjugate base of ethanoic acid, formed by the removal of a proton.
- Weak AcidAn acid that partially dissociates in solution, resulting in a relatively low concentration of hydrogen ions.
- Organic Acid-Base ChemistryThe study of acid-base reactions involving organic compounds, focusing on proton transfer.