Boron Family Reactions definitions Flashcards
 Back
BackBoron Family Reactions definitions
1/15
Terms in this set (15)
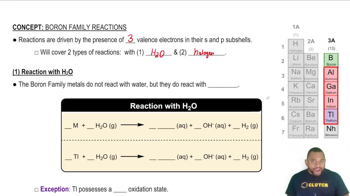
- Boron FamilyGroup 3A elements with three valence electrons, including boron and metals below it.
- Valence ElectronsElectrons in the outermost shell of an atom, crucial for chemical reactions.
- MetalloidAn element with properties intermediate between metals and nonmetals, like boron.
- Metallic BondingA type of chemical bonding that arises from the electrostatic attractive force between conduction electrons and positively charged metal ions.
- Covalent Network BondingA chemical bond where atoms are bonded by shared electrons in a continuous network.
- Oxidation StateThe degree of oxidation of an atom in a compound, indicating electron loss or gain.
- ThalliumA group 3A element with a preferred oxidation state of +1, differing from others in its group.
- Hydroxide IonA negatively charged ion (OH-) formed when group 3A elements react with steam.
- Hydrogen GasA diatomic molecule (H2) produced when group 3A elements react with steam.
- Ionic Halide SolidsCompounds formed when group 3A elements react with halogens, typically with formula MX3.
- HalogensGroup 7A elements that react with group 3A elements to form ionic compounds.
- StoichiometryThe calculation of reactants and products in chemical reactions, ensuring balanced equations.
- SteamThe gaseous state of water required for group 3A metals to react.
- Diatomic HalogenA molecule consisting of two atoms of a halogen, such as Cl2 or Br2.
- ChargeAn electrical property of particles, with group 3A elements typically having a +3 charge.