Intermolecular Forces and Physical Properties definitions Flashcards
 Back
BackIntermolecular Forces and Physical Properties definitions
1/10
Terms in this set (10)
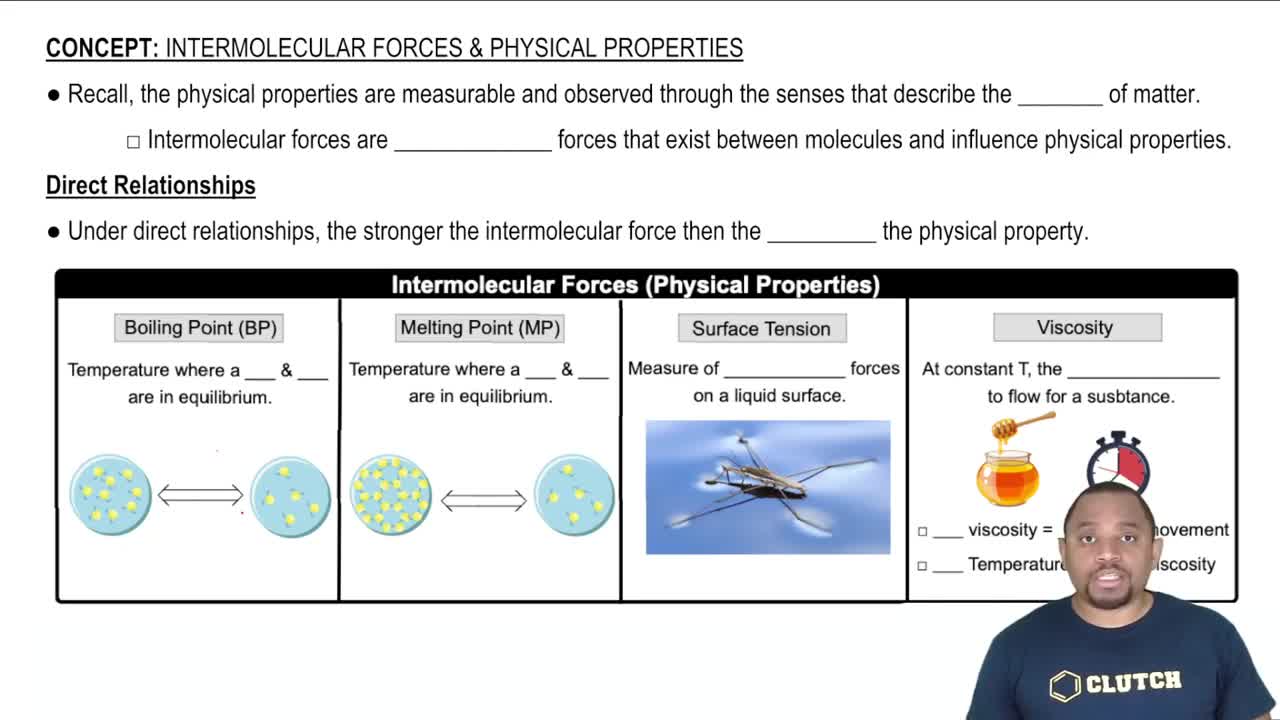
- Intermolecular ForcesAttractive forces between molecules influencing physical properties like boiling point and viscosity.
- Boiling PointTemperature where liquid and gas are in equilibrium; increases with stronger intermolecular forces.
- Melting PointTemperature where solid and liquid are in equilibrium; higher with stronger intermolecular forces.
- Surface TensionMeasure of cohesive forces at a liquid's surface; stronger with increased intermolecular forces.
- ViscosityResistance to flow in a substance; higher with stronger intermolecular forces, reduced by heat.
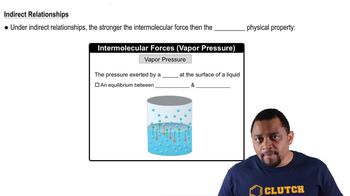
- Vapor PressurePressure exerted by gas on a liquid's surface; inversely related to intermolecular force strength.
- EquilibriumState where two phases, like liquid and gas, coexist without net change; crucial in boiling and melting points.
- Cohesive ForcesAttractive forces between similar molecules, contributing to surface tension.
- CondensationProcess where gas transforms into liquid, part of vapor pressure equilibrium.
- VaporizationProcess where liquid transforms into gas, part of vapor pressure equilibrium.