Body Centered Cubic Unit Cell definitions Flashcards
 Back
BackBody Centered Cubic Unit Cell definitions
1/11
Terms in this set (11)
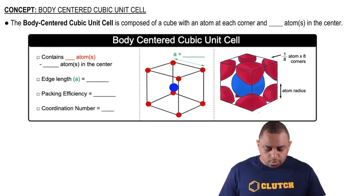
- Body-Centered CubicA crystal structure with atoms at each cube corner and one atom at the center, totaling two atoms per unit cell.
- Unit CellThe smallest repeating structure in a crystal lattice, defining the crystal's symmetry and dimensions.
- AtomThe basic unit of a chemical element, consisting of a nucleus surrounded by electrons.
- Corner AtomAn atom located at the corner of a unit cell, shared among adjacent cells, contributing partially to each.
- Central AtomAn atom located at the center of a body-centered cubic unit cell, not shared with other cells.
- Edge LengthThe distance between two adjacent corners of a unit cell, calculated as 4r/√3 for BCC.
- Atomic RadiusThe measure from the center of an atom's nucleus to its outermost electron shell.
- Packing EfficiencyThe fraction of volume in a crystal structure occupied by atoms, 68% for BCC.
- Coordination NumberThe number of nearest neighbor atoms surrounding an atom, 8 in a BCC structure.
- CrystallographyThe study of crystal structures and their properties, including unit cell arrangements.
- Lattice StructureThe regular, repeating arrangement of atoms in a crystal.