Benzene Reactions definitions Flashcards
 Back
BackBenzene Reactions definitions
1/14
Terms in this set (14)
- BenzeneAn aromatic compound known for its unique stability and tendency to undergo substitution reactions.
- AromaticityA property of cyclic, planar structures with delocalized pi electrons, contributing to stability.
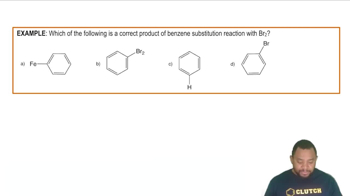
- Substitution ReactionA chemical reaction where an atom in a molecule is replaced by another atom or group.
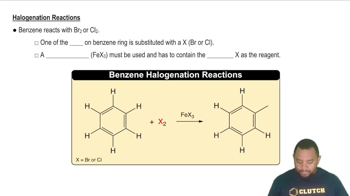
- HalogenationA reaction where a hydrogen atom on benzene is replaced by a halogen using a specific catalyst.
- Friedel-Crafts AlkylationA reaction where a hydrogen atom on benzene is replaced by an alkyl group using a catalyst.
- CatalystA substance that increases the rate of a chemical reaction without being consumed.
- Alkyl GroupA group derived from an alkane by removing one hydrogen atom.
- Alkyl HalideAn organic compound containing a halogen atom covalently bonded to an alkyl group.
- Pi BondsA type of covalent bond formed by the sideways overlap of atomic orbitals.
- FeBr3A catalyst used in the halogenation of benzene with bromine.
- FeCl3A catalyst used in the halogenation of benzene with chlorine.
- AlBr3A catalyst used in Friedel-Crafts alkylation with bromine-containing alkyl halides.
- AlCl3A catalyst used in Friedel-Crafts alkylation with chlorine-containing alkyl halides.
- MethylbenzeneA compound formed when a methyl group is substituted onto a benzene ring.