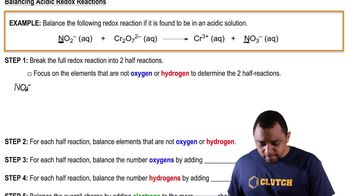
Balancing Redox Reactions: Acidic Solutions definitions Flashcards
 Back
BackBalancing Redox Reactions: Acidic Solutions definitions
1/10
Terms in this set (10)
- Redox ReactionA chemical process involving the transfer of electrons between two species, resulting in oxidation and reduction.
- OxidationThe process in which a reactant loses electrons, increasing its oxidation state.
- ReductionThe process in which a reactant gains electrons, decreasing its oxidation state.
- Acidic ConditionsA chemical environment characterized by the presence of H+ ions, affecting redox reactions.
- H+ IonA positively charged hydrogen ion, commonly present in acidic solutions.
- Half-ReactionA component of a redox reaction, representing either the oxidation or reduction process.
- Oxidation StateA number assigned to an element in a compound, indicating its degree of oxidation.
- Electron TransferThe movement of electrons from one reactant to another in a redox reaction.
- Charge BalanceThe principle of ensuring that the total charge is the same on both sides of a redox equation.
- Mass ConservationThe principle that mass is neither created nor destroyed in a chemical reaction.