Amine Reactions definitions Flashcards
 Back
BackAmine Reactions definitions
1/10
Terms in this set (10)
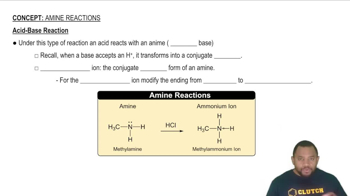
- AmineOrganic compounds derived from ammonia, acting as weak bases in reactions.
- Acid-Base ReactionA process where an acid donates a proton to a base, forming a conjugate acid.
- ProtonA positively charged subatomic particle, symbolized as H+, involved in acid-base reactions.
- Ammonium IonA positively charged ion formed when an amine accepts a proton, resulting in four nitrogen bonds.
- MethylamineA simple amine that reacts with acids to form methylammonium ions.
- Hydrochloric AcidA strong acid that donates a proton to bases like amines in reactions.
- Methylammonium IonThe product formed when methylamine accepts a proton, carrying a positive charge.
- Brønsted-Lowry DefinitionA theory describing acids as proton donors and bases as proton acceptors.
- Conjugate AcidThe species formed when a base gains a proton during an acid-base reaction.
- NitrogenAn element in amines that forms four bonds when converted to an ammonium ion.