Alcohol Reactions: Substitution Reactions definitions Flashcards
 Back
BackAlcohol Reactions: Substitution Reactions definitions
1/11
Terms in this set (11)
- AlcoholAn organic compound with a hydroxyl group (-OH) attached to a carbon atom.
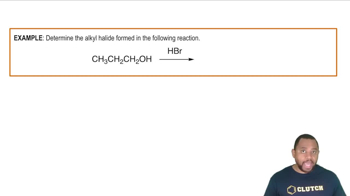
- Alkyl HalideA compound where a halogen atom replaces a hydroxyl group in an alcohol.
- Substitution ReactionA chemical reaction where an atom or group in a molecule is replaced by another atom or group.
- Hydrochloric AcidA strong acid used to convert alcohols into alkyl chlorides by replacing the hydroxyl group with chlorine.
- Hydrobromic AcidA strong acid used to convert alcohols into alkyl bromides by replacing the hydroxyl group with bromine.
- Hydroxyl GroupA functional group consisting of an oxygen atom bonded to a hydrogen atom (-OH).
- HalogenA group of elements including chlorine and bromine, used in substitution reactions with alcohols.
- ChlorineA halogen element that can replace the hydroxyl group in alcohols to form alkyl chlorides.
- BromineA halogen element that can replace the hydroxyl group in alcohols to form alkyl bromides.
- Functional GroupA specific group of atoms within a molecule responsible for characteristic reactions of that molecule.
- CarbonAn element that forms the backbone of organic molecules, bonding with hydroxyl groups in alcohols.