11. Bonding & Molecular Structure
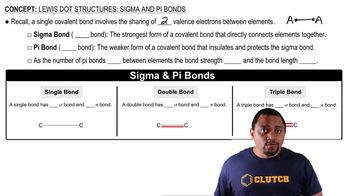
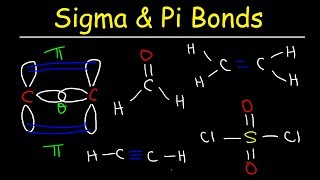
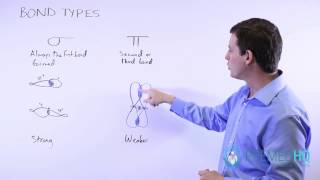
Lewis Dot Structures: Sigma & Pi Bonds
11. Bonding & Molecular Structure
Lewis Dot Structures: Sigma & Pi Bonds
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
How many pi bonds does the following molecule contain?
1362views3rank - Multiple Choice
How many sigma bonds does the following molecule contain?
2288views4rank - Multiple Choice
Which has greater bond strength between the carbon–carbon bond.
C2Cl2 vs. C2Cl6
1434views5rank1comments - Multiple Choice
Draw the total number of sigma and pi bonds of the sulfur trioxide molecule, SO3.
1435views4rank5comments - Open Question
Which of the two compounds, H2NNH2 and HNNH, has the strongest nitrogen-nitrogen bond, and which has the shorter nitrogen-nitrogen bond.
314views - Open QuestionHow many 𝜎 and 𝜋 bonds are in this molecule?658views
- Open Question
Which of the two compounds, H2NNH2 and HNNH, has the strongest nitrogen-nitrogen bond, and which has the shorter nitrogen-nitrogen bond?
319views - Open Question
How many sigma and pi bonds, respectively, are in this carboxylic acid?
558views