11. Bonding & Molecular Structure
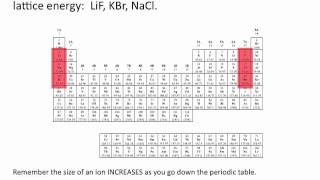
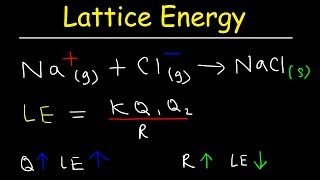
Lattice Energy
11. Bonding & Molecular Structure
Lattice Energy
Showing 8 of 8 videos
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
The lattice energy for ionic crystals decreases as the charge of the ions _____ and the size of the ions _____.
1035views - Multiple Choice
Which of the following compounds would you expect to have the highest boiling point?
864views - Multiple Choice
The solubilities of CaCrO4 and PbCrO4 in water at 25°C are approximately 0.111 g/L and 0.0905 g/L in H2O respectively. Based on this information, which compound do you think has the smaller lattice energy?
646views2rank - Multiple ChoiceWhich of the following reactions has ΔHrxn = ΔHlattice?397views
- Open Question
Consider the lattice energy of any ionic compound. what combination of ions and charges will produce the largest (in magnitude) lattice energies?
272views - Open QuestionWhich of the following trends in lattice energy is due to differences in ionic radii?356views
- Open QuestionRank the following ionic compounds by lattice energy.630views
- Open Question
MX(s) + crystal lattice energy → M+(g) + X- (g) is the reaction for crystal lattice energy.
350views