10. Periodic Properties of the Elements
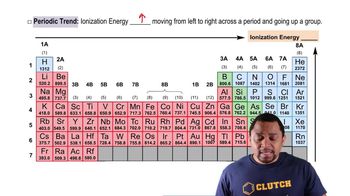
Periodic Trend: Ionization Energy
10. Periodic Properties of the Elements
Periodic Trend: Ionization Energy
Showing 5 of 5 videos
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple Choice
Rank the following elements in order of increasing ionization energy:Br, F, Ga, K and Se.
1504views4rank - Multiple Choice
Which of the following has the highest ionization energy?
2767views7rank - Multiple Choice
The energy of an electron in a one-electron atom or ion equals (–2.18 x 10–18 J) (Z2/n2). Estimate the ionization energy for the valence electron of the Li atom and compare it to its theoretical value of 520 kJ/mol.
2285views4rank4comments - Multiple ChoiceWhich of the following lists the atoms in order of increasing ionization energy (smallest to largest)?345views
- Open Question
Write a chemical equation representing the second ionization energy for lithium. Use e− as the symbol for an electron.
423views - Open Question
What is the reaction that corresponds to the first ionization energy of sodium, Na?
336views - Open Question
Order the elements S, Cl, and F in terms of increasing ionization energy.
323views - Open Question
Which best explains why ionization energy tends to decrease from the top to the bottom of a group?
281views