10. Periodic Properties of the Elements
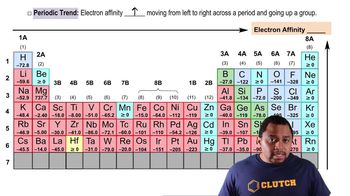
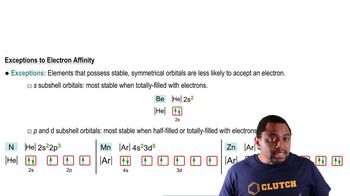
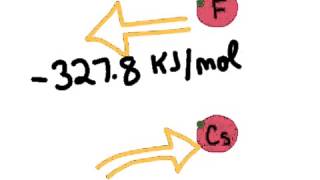
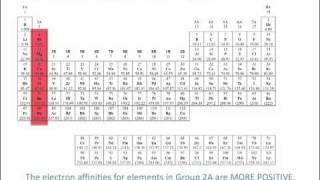
Periodic Trend: Electron Affinity
10. Periodic Properties of the Elements
Periodic Trend: Electron Affinity
Additional 7 creators.
Learn with other creators
Showing 10 of 10 videos
Practice this topic
- Multiple Choice
Which of the following represents the third electron affinity of Si?
718views3rank - Multiple Choice
Determine which atom in the following set has the most exothermic electron affinity:N, O, C, B, Ne
1009views - Multiple Choice
Rank the following elements in order of increasing electron affinity: Cs, Hg, F, S
1642views2rank4comments - Multiple ChoiceBased on general trends in electron affinity, which of the elements listed is most likely to have the most negative electron affinity?334views
- Open QuestionOf the following elements, ________ has the most negative electron affinity.437views
- Open QuestionWhich reaction below represents the second electron affinity of s?421views
- Open QuestionRank the following elements by electron affinity, from most positive to most negative ea value.472views
- Open QuestionWhich element has the highest (most negative) electron affinity?1035views