10. Periodic Properties of the Elements
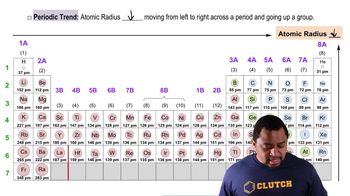
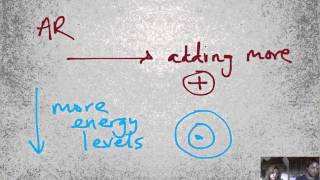
Periodic Trend: Atomic Radius
10. Periodic Properties of the Elements
Periodic Trend: Atomic Radius
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple Choice
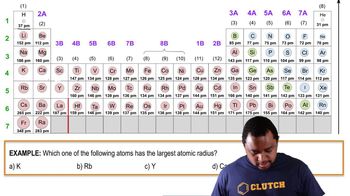
Arrange the following atoms in order of decreasing atomic radius:Sr, Se, Ne, Zn
2221views5rank - Multiple Choice
Which alkaline earth metal has the smallest atomic radius?
1561views8rank - Multiple Choice
In moving from top to bottom in the same column on the periodic table, what trend is expected for atomic size?
945views7rank - Multiple ChoiceIn which of the sets given are the atoms listed in order of increasing size (smallest to largest)?364views
- Open Question
What trend in size of the atom do you see as you move down a group?
288views - Open Question
Which element, oxygen (O) or fluorine (F), has a smaller atomic radius?
335views - Open Question
The experimental Bi−I bond length in bismuth triiodide, BiI3, is 2.81 Å. Based on this value and data in the figure, predict the atomic radius of Bi.
391views - Open QuestionAtomic radius generally increases as we move363views