9. Quantum Mechanics
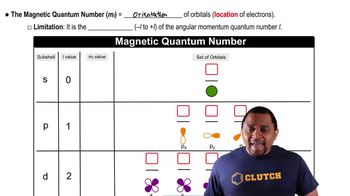
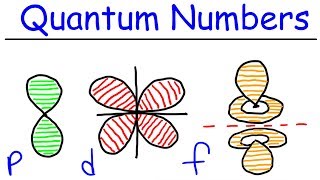
Quantum Numbers: Magnetic Quantum Number
Learn with other creators
Practice this topic
- Multiple Choice
How many different values of ml are possible for a 4d set of orbitals?
1331views5rank1comments - Multiple Choice
Select a correct set of values for an electron found within the designated 5d orbital.
829views1rank - Multiple Choice
Which of the following statements is false?
a) A set of d orbitals contains 5 orbitals.
b) A set of 4s orbitals would have more energy than a set of 3p orbitals.
c) The second shell of an atom possesses d orbitals.
d) A set of f orbitals contains 3 orbitals.
e) The first energy level contains only s orbitals.
1025views6rank2comments - Multiple ChoiceWhich sketch could represent an orbital with the quantum numbers n = 4 and l = 2?475views
- Open Question
What are the possible values of mℓ for an electron in a d orbital?
323views - Open Question
Suppose that, in an alternate universe, the possible values of ml were the integer values including 0 ranging from −l−1 to l+1 (instead of simply −l to +l). how many orbitals would exist in each of the following sublevels?
377views - Open QuestionWhat is the only possible value of mℓ for an electron in an s orbital?408views
- Open QuestionWhich set of quantum numbers cannot specify an orbital?471views