9. Quantum Mechanics
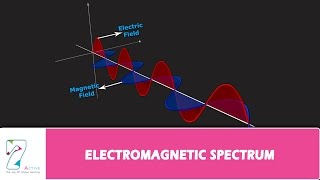
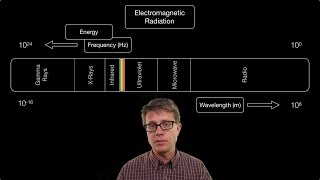
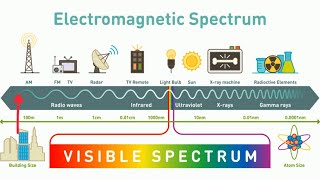
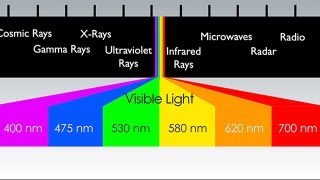
Electromagnetic Spectrum
9. Quantum Mechanics
Electromagnetic Spectrum
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Which of the following sources of electromagnetic radiation will have the highest frequency?
1527views3rank3comments - Multiple Choice
A carbon–oxygen double bond within a sugar molecule absorbs electromagnetic radiation at a frequency of 6.0 x 1012 s-1. What portion of the electromagnetic spectrum does this represent?
818views7rank - Multiple Choice
X-Ray detectors are devices that use scintillators to convert X-rays into light in order to detect X-Rays indirectly. Which of the following would be picked up by an X-Ray detector:radiation with a wavelength of 0.85 nm or a frequency of 6.52 x 1011 s-1?
1491views4rank3comments - Multiple ChoiceWhat frequency of light has a wavelength of 455 nm?484views
- Open Question
Arrange the following kinds of electromagnetic radiation in order of increasing wavelength: infrared, green light, red light, radio waves, x rays, ultraviolet light.
424views - Open QuestionDecreasing energy472views
- Open QuestionPlace the following types of electromagnetic radiation in order of increasing wavelength (shortest at the top to longest at the bottom).432views