8. Thermochemistry
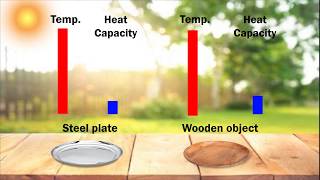
Heat Capacity
Learn with other creators
Practice this topic
- Multiple Choice
A sample of copper absorbs 3.53 kJ of heat, which increases the temperature by 25 ºC, determine the mass (in kg) of the copper sample if the specific heat capacity of copper is 0.385 J / g ºC.
1664views7rank - Multiple Choice
Based on their given specific heat capacities which substance would show the greatest temperature change upon absorbing 25.0 J of heat?
1600views6rank3comments - Multiple Choice
50.00 g of heated metal ore is placed into an insulated beaker containing 822.5 g of water. Once the metal heats up the final temperature of the water is 32.08 ºC. If the metal gains 14.55 kJ of energy, what is the initial temperature of the water?
1240views14rank3comments - Multiple ChoiceWhich of the following exchanges of energy has a positive sign for heat (relative to the system)?361views
- Open Question
If 14.5 kJ of heat were added to 485 g of liquid water, how much would its temperature increase?
351views - Open Question
The temperature of the cooling water as it leaves the hot engine of an automobile is 240 °F. After it passes through the radiator it has a temperature of 175 °F. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g °C.
267views - Open QuestionWhich sample is most likely to undergo the smallest change in temperature upon the absorption of 100 kj of heat?444views
- Open QuestionHow much heat is required to warm 1.50 kg of sand from 26.0 ∘c to 100.0 ∘c ?409views