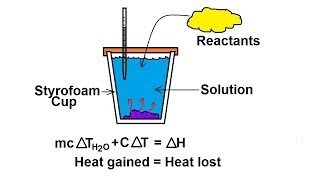
8. Thermochemistry
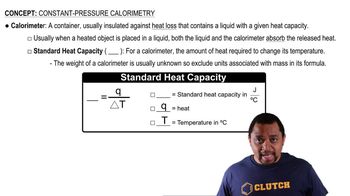
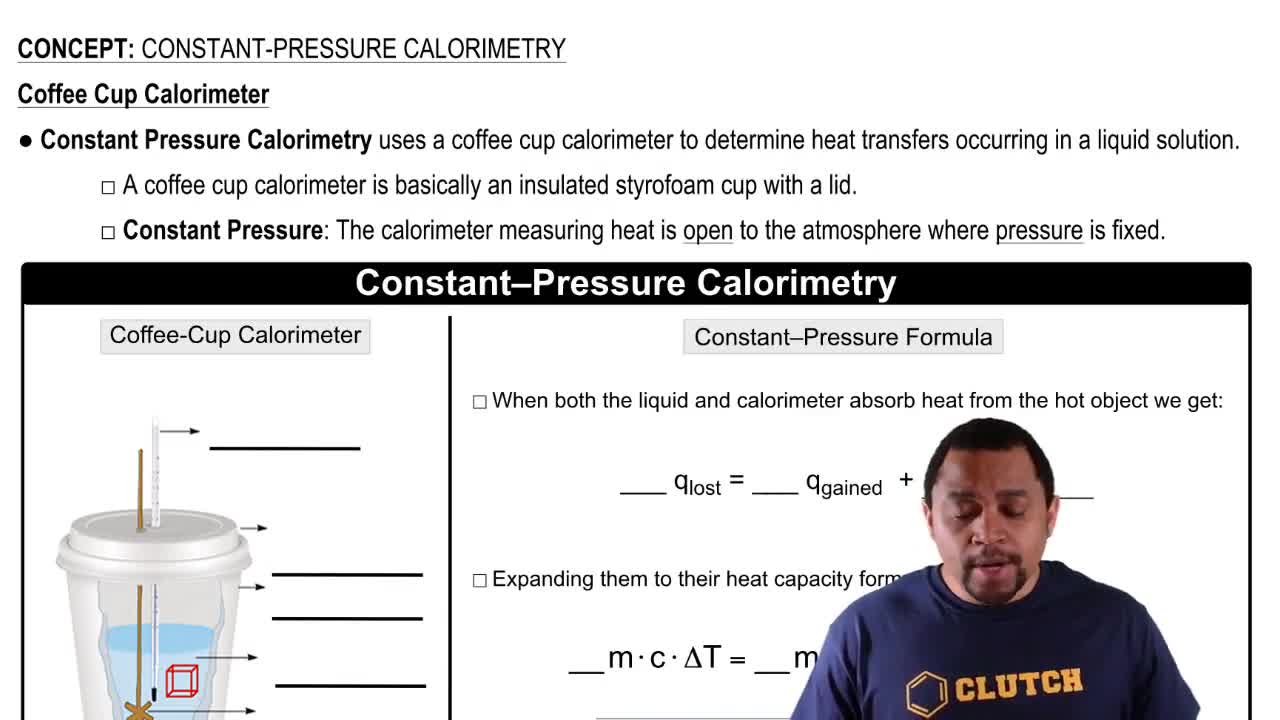
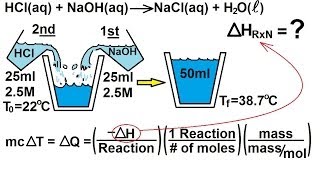
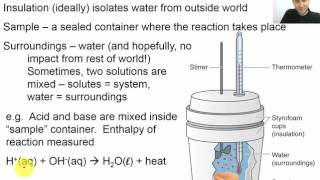
Constant-Pressure Calorimetry
8. Thermochemistry
Constant-Pressure Calorimetry
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
A 115.6 g piece of copper metal at 182.5 ºC is placed into 120.0 mL of methylene chloride at 31.0 ºC within a coffee-cup calorimeter. If the final temperature of the solution is 50.3 ºC, what is the specific heat of methylene chloride? Assume the calorimeter absorbs a negligible amount of heat. The specific heat of copper is 4.184 J/g ∙ ºC and the density of methylene chloride is 1.33 g/cm3.
987views1rank2comments - Multiple Choice
You place 75.0 mL of 0.100 M NaOH in a calorimeter at 25.00 ºC and carefully add 55.0 mL of 0.200 M HNO3, also at 25.00 ºC. After stirring, the final temperature is 53.35 ºC. Calculate the enthalpy ∆Hrxn (in J/mol) for the formation of water. (Specific heat capacity, Cs, and density of the solution:4.184 J/g∙K and 1.00 g/mL).
1298views6rank12comments - Multiple ChoiceWhen gasoline is burned at constant pressure, a certain amount of heat is emitted and work is done. Which of the following must be true?321views
- Open QuestionWhen a 4.25g sample of solid ammonium371views
- Multiple ChoiceA coffee-cup calorimeter contains 130.0 g of water at 25.2 °C. A 124.0-g block of copper metal is heated to 100.4 °C by putting it in a beaker of boiling water. The specific heat of Cu(s) is 0.385 J/g·K. The Cu is added to the calorimeter, and after thermal equilibrium is reached, the final temperature of the system is measured. What is the final temperature of the water in the calorimeter?2views