7. Gases
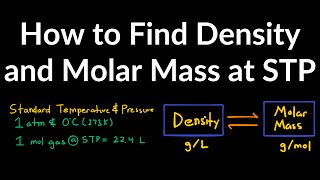
The Ideal Gas Law: Molar Mass
7. Gases
The Ideal Gas Law: Molar Mass
Showing 5 of 5 videos
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
To identify a homonuclear diatomic gas, a chemist weighted an evacuated flask with a volume of 3.9 L then filled it with the gas at a pressure of 2.00 atm and 29.0 ºC. The chemist then re-weighted the flask and recorded the difference in mass as 8.81 g. Identify the gas.
675views1rank - Multiple Choice
What is the molecular formula of a compound that contains 39.0% carbon, 16.0% hydrogen, and 45.0% nitrogen, if 0.1576 g of the compound occupies 125 mL with a pressure of 0.9820 atm at 295.15 K?
629views1rank1comments - Multiple ChoiceCalculate the molar mass for 2.50 g of an unknown gas in a 0.500-L container at a pressure of 735 mmHg and a temperature of 22℃.506views
- Multiple ChoiceA 0.465 g sample of an unknown compound occupies 245 mL at 298 K and 1.24 bar. What is the molar mass of the unknown compound?