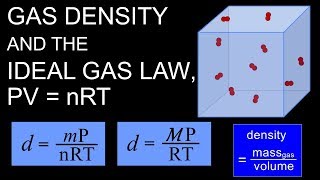
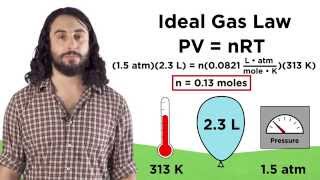
7. Gases
The Ideal Gas Law Derivations
Learn with other creators
Practice this topic
- Multiple Choice
PRACTICE: A sample of nitrogen dioxide gas at 130 ºC and 315 torr occupies a volume of 1750 mL. What will the gas pressure be if the volume is reduced to 320 mL at 130 ºC?
822views9rank1comments - Multiple Choice
A cylinder with a movable piston contains 0.615 moles of gas and has a volume of 295 mL. What will its volume be if 0.103 moles of gas escaped?
1016views6rank - Multiple Choice
On most spray cans it is advised to never expose them to fire. A spray can is used until all that remains is the propellant gas, which has a pressure of 1350 torr at 25 ºC. If the can is then thrown into a fire at 455 ºC, what will be the pressure (in torr) in the can?
784views8rank - Open Question
How many 3-liter balloons could the 12-L helium tank pressurized to 160 atm fill? Keep in mind that an "exhausted" helium tank is not empty. In other words, once the gas inside the tank reaches atmospheric pressure, it will no longer be able to fill balloons.
410views - Open Question
A sample of nitrogen gas at 298 K and 745 torr has a volume of 37.42 L. What volume will it occupy if the pressure is increased to 894 torr at constant temperature?
319views - Open Question
To what temperature must a balloon, initially at 25°C and 2.00 L, be heated in order to have a volume of 6.00 L?
354views - Open Question
A syringe contains 0.65 moles of He gas that occupy 750.0 ml. What volume (in l) of gas will the syringe hold if 0.35 moles of ne is added?
332views