7. Gases
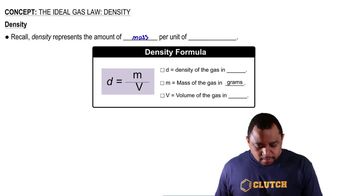
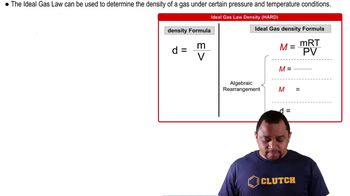
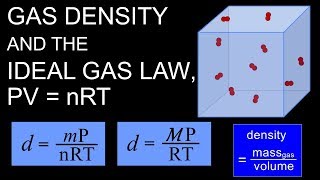
The Ideal Gas Law: Density
Learn with other creators
Practice this topic
- Multiple Choice
Consider two containers of gases at the same temperature. One has helium at a pressure of 1.00 atm. The other contains carbon dioxide with the same density as the helium gas. What is the pressure of the carbon dioxide gas sample?
663views1rank - Multiple Choice
Determine the molecular formula of a gaseous compound that is 49.48% carbon, 5.19% hydrogen, 28.85% nitrogen, and 16.48% oxygen. At 27°C, the density of the gas is 1.5535 g/L and it exerts a pressure of 0.0985 atm.
927views1rank2comments - Multiple ChoiceCalculate the density of argon gas at 22.5℃ and a pressure of 2.0 atm.665views
- Open Question
What is the density of laughing gas, dinitrogen monoxide, N2O, at a temperature of 325 K and a pressure of 113.0 kPa?
372views - Open Question
a gas with a molar mass of 26.54 g/mol is at a pressure of 172 torr and 27 °C. What is its density?
264views - Open Question
The density of a gas is 2.49 g/L at 3.00 atm and 25oC. Calculate the molar mass of the gas.
355views - Open Question
What is the molar mass of an unknown gas with a density of 3.55 g/L at 2.00 atm and 55.0 °C?
356views - Multiple ChoiceAt what temperature in Kelvin does sulfur hexafluoride (SF6) have a density of 0.5550 g/L at a pressure of 0.8210 atm?