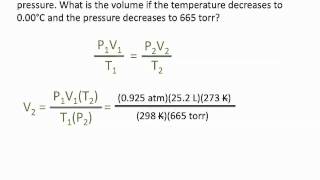
7. Gases
Standard Temperature and Pressure
7. Gases
Standard Temperature and Pressure
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
A sample of dichloromethane gas (CH2Cl2) occupies 32.6 L at 310 K and 5.30 atm. Determine its volume at STP?
872views3rank1comments - Multiple Choice
Which gas sample has the greatest volume at STP?
2425views1rank - Multiple Choice
Nitrogen and hydrogen combine to form ammonia via the following reaction:
1 N2 (s) + 3 H2 (g) → 2 NH3 (g)
What mass of nitrogen is required to completely react with 800.0 mL H2 at STP?19600views1rank2comments - Multiple ChoiceWhat is the pressure inside a 1.5-L container that is charged with 3.0 moles of a gas at 35.0℃?483views1rank
- Open Question
Approximately how many moles of Kr+ are contained in the laser tube at 0°C and 1 atm?
473views - Open Question
What is the density of xe gas at stp
466views - Open Question
Use the molar volume of a gas at STP to calculate the density (in g/l) of nitrogen gas at STP.
295views - Open Question
At STP, what is the volume occupied by 33.5 g of argon gas?
397views