7. Gases
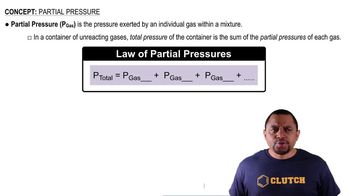
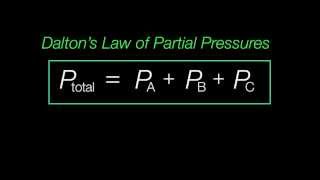
Partial Pressure
Learn with other creators
Practice this topic
- Multiple Choice
A sample of 3.51 g argon and an unknown amount of oxygen are mixed in a container at room temperature. The partial pressure of argon was calculated as 71.0 torr and the partial pressure of oxygen as 188 torr. What is the mass of the oxygen within the container?
913views2rank2comments - Multiple Choice
A gas mixture contains 72.8% chlorine and 27.2% neon by mass. What is the partial pressure of neon in the mixture if the total pressure is recorded as 809 mmHg?
1164views3rank - Multiple ChoiceA 2.50-L container contains a mixture of helium, nitrogen, and argon gas. The total pressure is 1.800 atm at 295 K. The partial pressure of helium is 0.650 atm, and the partial pressure of nitrogen is 0.350 atm. How many moles of argon gas are present in the mixture?476views
- Multiple ChoiceIn the reaction of calcium metal and water, 50.0 mL of hydrogen gas is collected over water at 25.0℃ with a total pressure of 752 mmHg. Calculate the number of moles of hydrogen gas that was produced.382views
- Open Question
What is the mole fraction of oxygen in a gas mixture that is 37% oxygen and 63% nitrogen by volume?
336views - Open Question
A 15-l cylinder contains 4.0 g of hydrogen and 28 g of nitrogen. If the temperature is 27 °C, what is the total pressure of the mixture?
329views - Open Question
A mixture of three gases has a total pressure of 1,380 mmHg at 298 K. The mixture is analyzed and is found to contain 1.27 mol CO2, 3.04 mol CO, and 1.50 mol Ar. What is the partial pressure of Ar?
364views - Open Question
A mixture of 10.0 g of nNe and 10.0 g Ar have a total pressure of 1.6 atm. What is the partial pressure of Ne?
555views